Lead to Candidate Design
A case study on Lead to candidate design: a Phase II clinical compound
PH-797804, a p38 MAP kinase inhibitor, is in Phase II trials for treating inflammation. The initial high-throughput screening (HTS) hit candidate was SC-25028, which demonstrated a promising profile: µM potency against p38, selectivity against panel of kinases (200), but suffered from poor metabolic stability [2]. To tackle this challenge, the authors have meticulously designed over 80 analogues using their medicinal chemistry and modelling skills. This interdisciplinary team addressed metabolic stability issues in a systematic way while improving the potency, and selectivity, leading to the development of PH-797804. (Figure 1)[2–4].
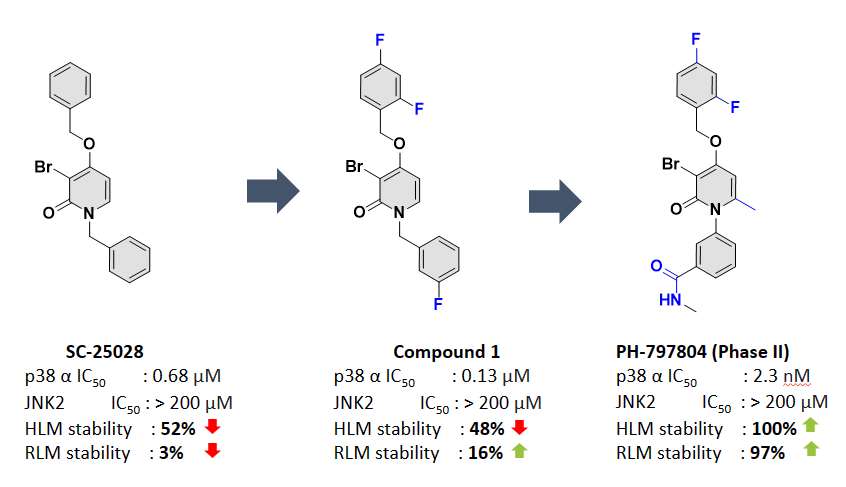
Figure 1: Depicts the medicinal chemistry optimization efforts on the initial HTS hit (SC-25028) to the development of the candidate PH-797804 to address the potency, metabolic stability, and pharmacokinetics profiles.
The hit candidate, SC-25028, was substantially metabolized: only 52% of this compound was detected after 45 minutes of incubation. The initial hypothesis was that unsubstituted aromatic rings were sites of metabolism. We questioned whether IMPACTS could generate hypotheses aligned with the thought processes of medicinal chemists and potentially follow a similar ideation path.
We asked IMPACTS to predict the top five most likely SoMs for SC-25028 using all the CYP models available in the current version, since it is unclear which CYP is most likely to metabolize the compound. After each Impacts run, the top 5 SoMs are generated as requested, along with the activation energies, reaction types, and transition states of the molecule reacting with CYPs. This information is vital to understanding potential metabolic sites and informing subsequent design decisions.
IMPACTS Predictions:
IMPACTS predicted O-dealkylation at the methylene group at the benzylic position of the benzyloxy moiety as a primary SoM as shown in Figure 2. Aromatic oxidation was predicted as next potential metabolic sites on both unsubstituted aromatic rings of the compound. These IMPACTS predictions closely matched the hypotheses of medicinal chemists. According to the literature, researchers conducted optimization studies that were specifically focused on these identified sites.
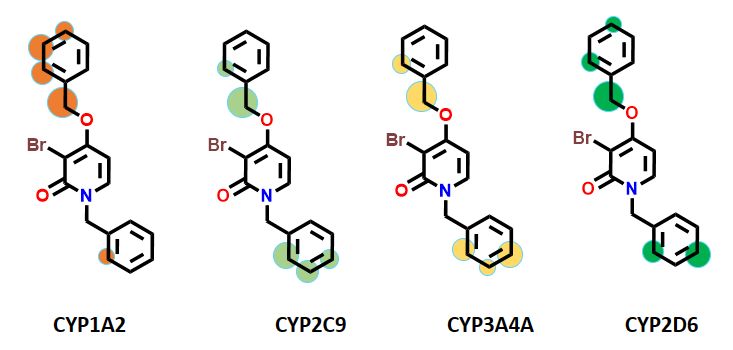
Figure 2: IMPACTS site of metabolism (SoM) prediction for SC-25028. SoMs are defined as circles, sized to indicate priority: larger circles for top priority sites and smaller circles for less critical ones. Each CYP is denoted by unique colour codes.
All CYP models predicted that the benzyloxy moiety’s methylene group was a top SoM, indicating O-dealkylation. Literature data indicate that researchers explored only ethylene and benzylamine linkers at this position and found these modifications to be less effective. Authors mentioned that the benzyloxy moiety was found to be ideal substitution for occupying the lipophilic pocket beyond the gatekeeper residue, Thr103. This position hasn’t been explored from the perspective of metabolic stability, so we cannot compare its impact of SoM on metabolic stability. (Figure 3).
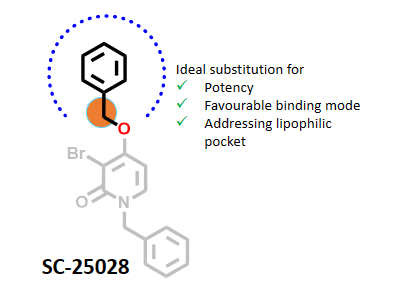
Figure 3: IMPACTS predicted that the methylene group in the benzyloxy moiety is a potential metabolic hot spot (SoM), whereas this group was found to be an ideal substitution and remained unchained during the lead optimization.
Various mono and di-substitutions were explored for the 2′, 4′-position of the benzyloxy group, including halogens (Cl, and F). In terms of metabolic stability and selectivity, 2,4-difluorobenzyloxy was found to be the most effective modification. This medicinal chemistry hypothesis and efforts were very well aligned with IMPACTS predictions. The use of IMPACTS would have been very helpful in understanding hot spots (SoMs) and in decision making (Figure 4).
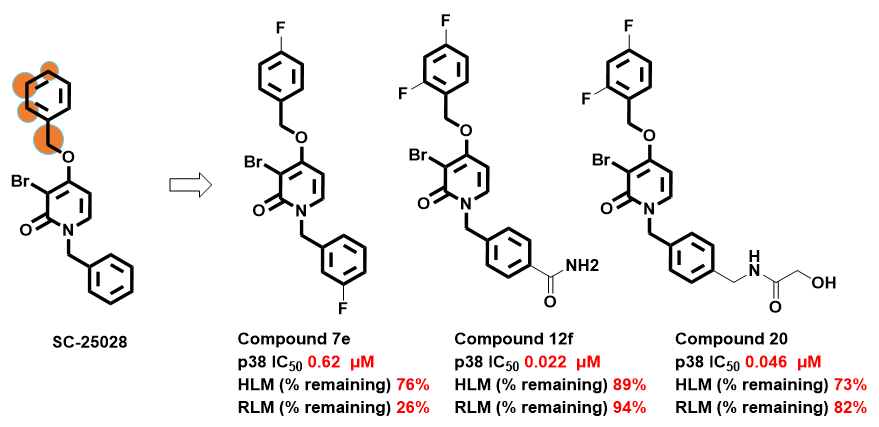
Figure 4: IMPACTS CYP1A2 model prediction highlighting the SoMs on benzyloxy moiety and representative substitutions explored around benzyloxy group for metabolic stability concerns [2]. Here, we captured exclusively CYP1A2 predictions since the authors did not specify which CYP enzyme is responsible for metabolizing the compound. The predictions for all four CYP enzymes indicated either the 2’ or 4’ positions, or both, as sites of metabolism (SoMs), as illustrated in Figure 2.
As a next step, authors replaced N-benzyl group with N-phenyl to tackle metabolic stability and to design more selective inhibitors for p38. Various mono, and di-substitutions were explored on the N-phenyl moiety with a focus on improving selectivity and metabolic stability by introducing polar groups on 3′, 4′ positions of the N-phenyl to engage Asp112 and Asn115. Medicinal chemistry iterations identified the 3′-CONHMe substitution as the most promising, ultimately leading to the discovery of PH-797804 as a clinical candidate. In Figure 5, IMPACTS predictions for SC-25028 (by CYP3A4A model) and compound 2 are well aligned with medicinal chemistry plans. As we did not observe any SoMs on N-phenyl groups from other CYP models, we captured CYP2D6 predictions for compound 2.
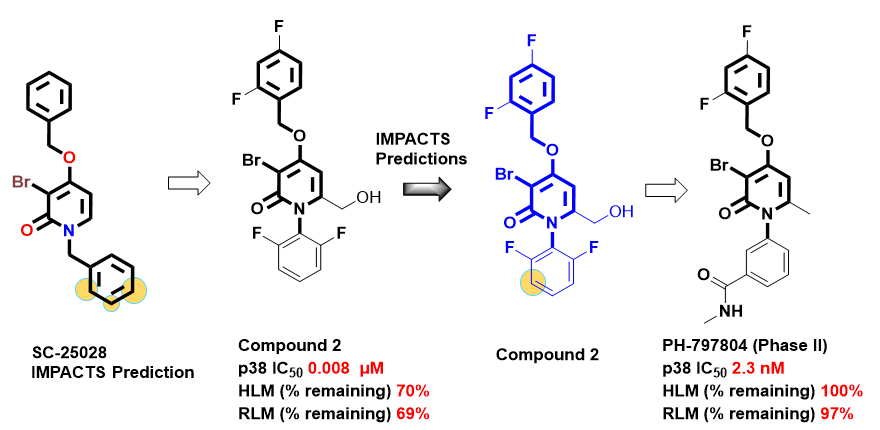
Figure 5: IMPACTS prediction on SC-25028 and compound 2 highlighting the SoMs on N-benzyl and N-phenyl moiety and representative substitutions explored around this group to address metabolic stability concerns.2,3 Here, we captured CYP3A4 model predictions for SC-25028, and observed similar or same SoMs, as illustrated in Figure 2. For compound 2, we observed SoMs on N-phenyl group by CYP2D6, and no such predictions observed on this group by other 3 CYP models. So, we captured CYP2D6 predictions for compound 2.
In conclusion, this retrospective case study illustrates how IMPACTS SoMs enhance decision-making for initial candidates by closely matching medicinal chemistry strategies. These tools can speed up discovery and reduce the need for extensive synthesis and screening. Predictive analytics can make drug discovery more efficient, improving the decision-making process and fast-tracking development from concept to candidate.
VALUE ADDITIONS:
Predictive tools like IMPACTS enhance strategic planning in drug design projects by enabling:
-
- A data-driven foundation for decision-making
- Early identification of potential metabolic concerns
- The design of lead candidates with higher likelihood of development success
- Significant reduction in development costs by minimizing extensive synthesis and screening (e.g. the 80+ compounds in this retrospective case study)
References:
-
- Campagna-Slater, V.; Pottel, J.; Therrien, E.; Cantin, L.-D.; Moitessier, N. Development of a Computational Tool to Rival Experts in the Prediction of Sites of Metabolism of Xenobiotics by P450s.
- Selness, S. R.et al., Discovery of N-Substituted Pyridinones as Potent and Selective Inhibitors of P38 Kinase.
- Selness, S. R. et al., Design, Synthesis and Activity of a Potent, Selective Series of N-Aryl Pyridinone Inhibitors of P38 Kinase.
- Selness, S. R. et al., Discovery of PH-797804, a Highly Selective and Potent Inhibitor of P38 MAP Kinase
