The Impact of IMPACTS
A blog series on retrospective case studies in metabolic stability prediction and optimization studies
Metabolic stability is a crucial factor in drug development, as it directly influences the efficacy and safety of a pharmaceutical candidate. Metabolic instability in drugs can cause rapid degradation, leading to low therapeutic levels and/or formation of harmful metabolites. A substantial number of lead candidates have failed or had their development halted due to metabolic stability issues; while some drugs have been withdrawn from the market.
Did you know:
-
- Sites of Metabolism (SOMs) or ‘hot spots’ refer to locations within a molecular structure where metabolic enzymes act to modify the compound.
- Identifying and addressing metabolic ‘hot spots’ is key.
- These ‘hot spots’ are areas within the compound that are most susceptible to metabolic breakdown by the body’s enzymes, predominantly in the liver.
- The liver’s cytochrome P450 (CYP) enzymes are responsible for the breakdown of many drugs, making them primary targets for investigations.
Our proprietary software, IMPACTS, is a user-friendly tool for site-of-metabolism (SoM) identification of xenobiotics. Impacts combines docking to cytochrome P450 (CYP) enzymes, transition state modelling, and rule-based substrate reactivity prediction.
IMPACTS has been tested on over 700 molecules across seven different CYP isoforms (1A2, 2A6, 2B6, 2C8, 2C9, 2D6, 3A4), with accuracies ranging from 74% (CYP2A6 and CYP2B6) to 82% (CYP1A2) when the top 2 SoMs are considered[1]. Testing on isoforms CYP2C19 and CYP2E1 is currently ongoing, which will ensure that IMPACTS will be tested on over 97% of the CYP isoforms involved in the metabolism of xenobiotics.
The purpose of this blog series is to provide retrospective case studies on metabolic stability, focusing on how IMPACTS can be used to identify and interpret SoMs for a given compound. These predictions can guide medicinal chemists to design more stable molecules.
These case studies are derived from the scientific literature, including lead optimization and lead-to-candidate identification studies, with an emphasis on metabolic stability. We will showcase how Impacts identifies ‘hot spots’ and how the predictions align with experimental data.
⇒ PH-797804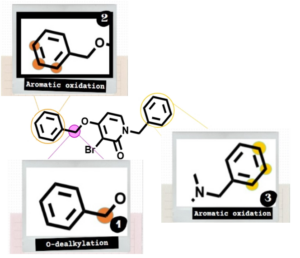
⇒ Phase II clinical compound
⇒ Kinase inhibitor
⇒ Metabolic stability optimization
Read more about this case study: Lead to candidate design
