Alchem Laboratories
In 2022, we completed research within our multi-year collaboration with Alchem Laboratories Corporation (United States). For three years, we provided computational expertise to guide the design of novel therapeutics targeting multiple protein classes involved in critical biological functions. Some of our work is summarized below:
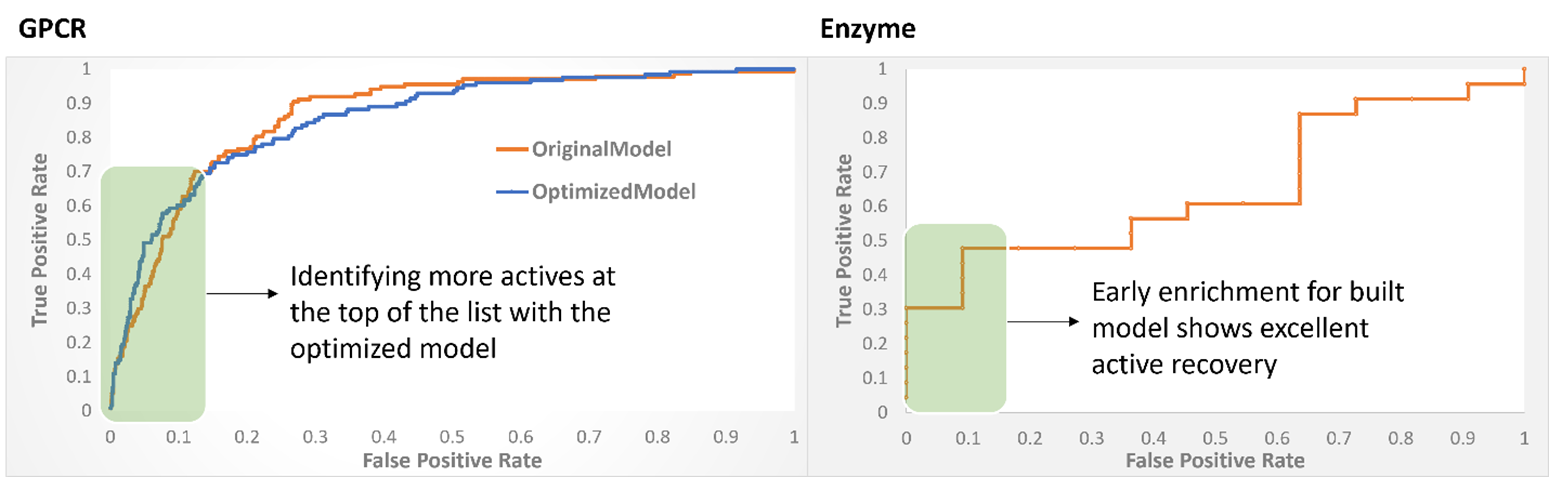
The proteins of interest (one GPCR and one enzyme) had a dearth of structural information available for us to sift through. The enzyme presented an added challenge of having to be targeted covalently. We benchmarked protein models that we constructed, and the applicability of our docking software Fitted using reported structural and functional experimental observables.
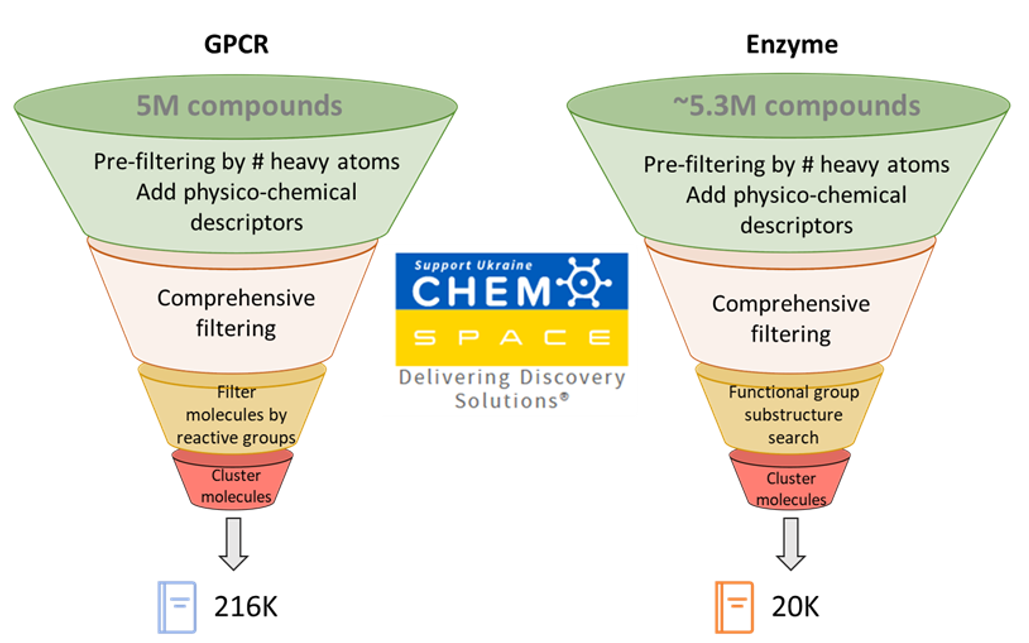
From a chemistry perspective, we developed libraries for virtual screening: some derived from ~5M compounds provided by our trusted partner Chemspace and some virtually generated for custom synthesis. We used our drug discovery platform Forecaster to add over 40 physico-chemical descriptors (molecular weight, hydrogen bond donors/acceptors, logP, etc.) to these molecules and to filter the libraries using common medicinal chemistry guidelines. We clustered and focused on diversity and similarity when appropriate.
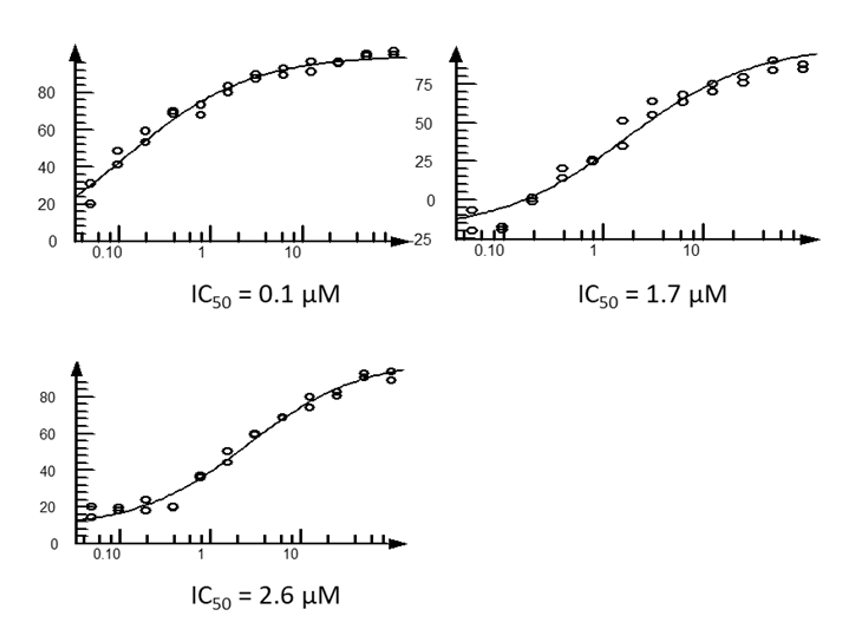
For the GPCR, through visual and data analysis we identified 45 compounds for testing after initial virtual screening. We achieved a 20% hit-rate: nine sub-10 µM compounds, including a mid-low nanomolar antagonist (experimentally tested in dose-response, multi-replicate assays). Some of these compounds serendipitously showed selectivity against other members of the protein family and most are structurally novel compared to reported ligands. Our computer-aided drug design (CADD) approach facilitated optimization iterations and the most promising compounds are being tested in more advanced experimental assays.
For our enzyme, we identified 60 compounds for testing after an initial virtual screening. A lack of reactivity (covalent binders) hindered the utility of the compounds. We incorporated new experimental and theoretical knowledge into our modeling and designed a 6-fold improvement on the current best-in-class compound targeting multiple enzymatic states—a key property missing from the current standard of care.
In both programs, compounds showed strong activity and excellent ADMET properties in vitro and efficacy, single-dose toxicity, and pharmacokinetic in vivo studies. The lead compound of one of the programs is scheduled to start preclinical GLP toxicology studies in 2024.
Throughout our collaboration, the knowledge transfer between our teams has been key to its success. Mutual support for pursuing various research avenues have borne fruit in identifying novel compounds that act on proteins involved in critical pathways. In addition, such projects highlight the value of CADD and the cost-benefit to achieving desired results compared to solely experimental approaches.
Combining MFI’s software, service, and expertise made them an excellent partner for our DoD project. We are excited by the results thus far (nanomolar compounds) and look forward to continuing our relationship.
– Dr. James Talton, Alchem Laboratories